
Limestone
the raw material of Heavy Calcium Carbonate

Near "TAISHAKU-KYOU", there are the mines of the company which I work for. They have a lot of crystalline limestone of good quality.
So that is useful for stable supply of the Heavy Calcium Carbonate products of good quality and enough quantity.
In this page, I explain about the Limestone, the raw material of Heavy Calcium Carbonate.
On the theory, Calcium Carbonate consists of 56% of Calcium Oxide[CaO] and 44% of Carbon Dioxide[CO2]. Because the limestone is natural thing, it contains a little impurities like magnesium, aluminum, silicic acid or iron, etc...
It is said that the ore deposits of limestone in Japanese Islands, for example "TAISHAKU-DAI", were made by the following process;
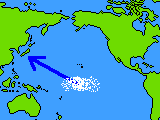 About 3 hundred million years ago, there were the remains of living things accumulating under the sea around the equator of the central Pacific ocean.
About 3 hundred million years ago, there were the remains of living things accumulating under the sea around the equator of the central Pacific ocean.
They moved little by little to north-west direction by the movement of the earth's crust and appeared in the present location of Japanese Islands by colliding with the Asian continent's crust and rising.
When they were rising from the sea, they were rapidly heated by contacting with heat source like magma gushing out from volcanoes.
At that time, the impurities in the remains were get away and they became the ore deposits of pure limestone.
The ore deposit of limestone near the surface contacting to heat source became the crystalline limestone of higher purity.
On the other hand, the ore deposit of limestone far from the heat source or heat source of low temperature became non-crystalline limestone of lower purity.
The Heavy Calcium Carbonate is produced from the crystalline limestone.
 There was old concern between human race and limestone. Huge limestones were used for the pyramids in ancient Egypt and for the shrines in ancient Greek or Roman times.
There was old concern between human race and limestone. Huge limestones were used for the pyramids in ancient Egypt and for the shrines in ancient Greek or Roman times.
Å@In addition, many old stone statues and sculptures were made with the Marble, a kind of the limestone. The Marble have been made use of the interior and exterior of buildings since acient times.
It was after the Industrial Revolution that the limestone became used in large quantity.
It increased by growing of the cement industories which use the limestone as its raw material and of the steel industries which use the limestone to remove impurity from ironstone.
 As the development of the petrochemical industries after the World War II, the Calcium Carbonate which is made from the limestone have been used for the various purposes like the filler of plastic, the increaser or processing helper of rubber, the raw material of paint, the additive in paper, the calcium medicine and the agricultural chemical, etc...
As the development of the petrochemical industries after the World War II, the Calcium Carbonate which is made from the limestone have been used for the various purposes like the filler of plastic, the increaser or processing helper of rubber, the raw material of paint, the additive in paper, the calcium medicine and the agricultural chemical, etc...
